Compliance
Compliance-Related Initiatives
As an R&D-oriented pharmaceutical company, Kissei Pharmaceutical strives to contribute to society through high-quality, innovative pharmaceutical products. Furthermore, as a standing director company of the Japan Pharmaceutical Manufacturers Association (JPMA), a company listed on the Tokyo Stock Exchange Prime Market, and a responsible pharmaceutical company, we are expected to maintain an open corporate culture towards the international community and to serve as a role model for society. We recognize that management in accordance with the law is the foundation and prerequisite of all corporate activities in order to realize our philosophy and meet expectations. Based on this recognition, we have formulated the Kissei Code of Conduct, which serves as a code of conduct for corporate activities, as well as our Compliance Program Manual, and use both to ensure compliance with laws and high ethical standards in our operations.
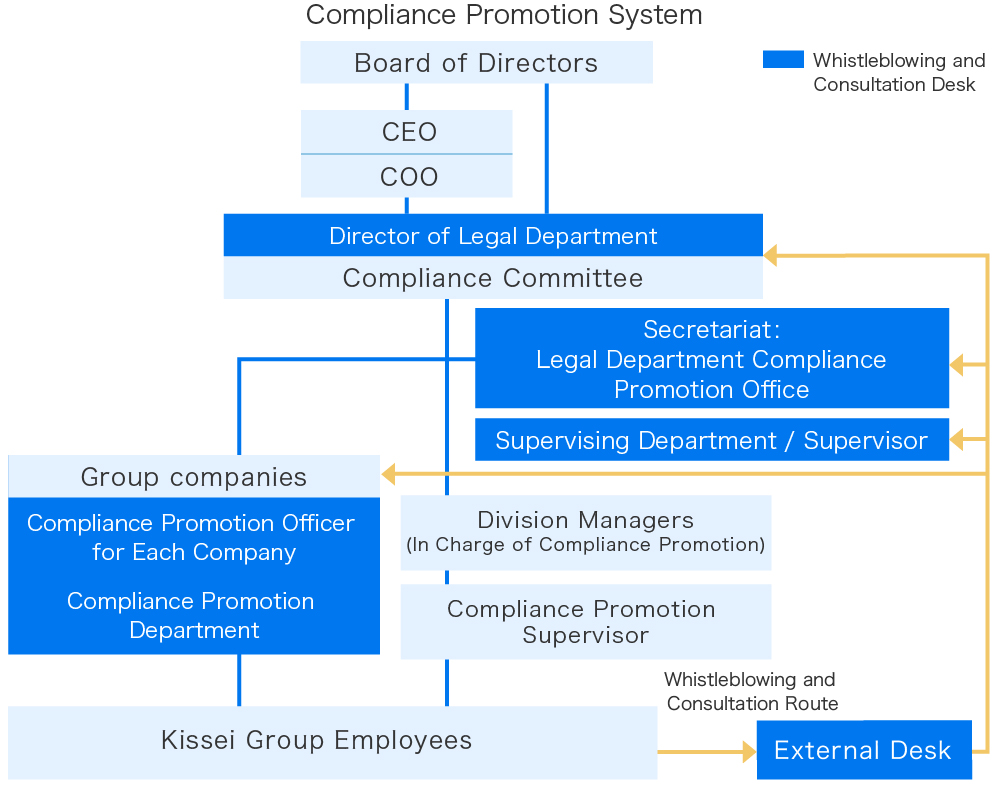
Compliance Promotion System
Kissei Pharmaceutical has established a Compliance Committee, commissioned by the Board of Directors, to promote the management in accordance with the law.
The committee, chaired by the director of the Legal Department and composed of the heads of each department, deliberates and decides on the concrete implementation plans for the annual Compliance Program in order to foster a high sense of ethics and ensure thorough compliance. Once finalized, these action plans are implemented thoroughly by each department head. Additionally, the Compliance Promotion Office of the Legal Department organizes and manages the compliance program, formulates implementation plans, and conducts awareness activities such as education and training.
The Kissei Group also engages in Groupwide compliance practices led by compliance officers appointed by each Group company under an internal control agreement. The Kissei Group Compliance Officers’ Meeting is held regularly, during which these compliance officers share action plans, report results, and exchange related information. At these meetings, compliance officers also receive education and training.
Whistleblowing and Consultation System (Kissei Hotline)
The Kissei Group has established a reporting and consultation system called the "Kissei Hotline" in accordance with the Act for Partial Revision of the Whistleblower Protection Act (Act No. 51 of 2020). Under this system, we aim to protect whistleblowers and contribute to the prevention and containment of legal violations, as well as damage or loss within our group. Through these efforts, we strive to foster a high level of organizational autonomy that possesses self-purifying capabilities to address legal violations and similar issues within the company.
Officers, employees, and people who have left the company can file a report or consult with an external contact independent of the Company regarding legal violations or harassment within the Group. The external contact can be reached by phone, email, post, or via a dedicated website. Furthermore, users of the hotline can opt to remain anonymous, in which case the Company will not know who is filing the report.
Initiatives for Responsible Product Supply and Sales Information Provision Activities
Kissei Pharmaceutical recognizes its important mission (challenge) as an R&D-oriented pharmaceutical company to support people's health, and undertakes necessary efforts daily. Specifically, by engaging in the research and development of safe and effective pharmaceuticals, manufacturing high-quality products and ensuring their stable supply, promoting sales activities in compliance with regulations, and providing appropriate information required in medical settings, we aim to address the challenges in the manufacturing and sales of pharmaceuticals and contribute to society through high-quality, innovative pharmaceutical products.
As part of this effort, we have established the "Kissei Code of Practice" and the "Kissei Code of Practice for the Promotion of Ethical Drugs" as our own codes, in accordance with the JPMA Code of Practice set by the JPMA based on international industry standards, and we adhere to these codes as norms for interactions between all our officers and employees and healthcare professionals, medical institutions, and patient organizations.
Furthermore, to provide healthcare professionals with the necessary information about pharmaceuticals, we comply with the relevant regulations such as the "Guidelines for Sales Information Provision Activities for Ethical Drugs" and strive to provide scientifically based and timely, and appropriate information. Additionally, to ensure fair and honest sales information provision activities for pharmaceuticals, we conduct material reviews and monitoring of sales information provision activities.
Compliance Promotion Activities (management in accordance with the law)
Kissei Pharmaceutical recognizes that it is a fundamental premise for executives and employees to comply with laws and internal regulations in their daily activities. Additionally, it is crucial to adhere to corporate ethics that support this compliance. Each individual will reliably fulfill their roles and responsibilities in the management in accordance with the law. Therefore, continuous education and training are essential and indispensable.
We distribute a "Compliance Program Manual" to all executives and employees, in addition to regular directives from top management regarding the management in accordance with the law, to serve as guidelines for appropriate conduct and actions in their daily activities. This initiative aims to enhance understanding and awareness of compliance. Additionally, in conjunction with the annual Pharmaceutical Affairs Code promotion month of JPMA, we conduct regular training on the "Kissei Code of Practice" for all executives and employees. Furthermore, we continuously provide education on the Guidelines for Sales Information Provision Activities for Ethical Drugs, thereby striving to ensure responsible development, manufacturing, supply, sales information provision activities, and information dissemination of pharmaceuticals.
In addition, the Company provides rank-based education to officers, division and department managers, newly appointed managers and supervisors, and new employees. This education matches the relevant stage in their careers. Furthermore, employees receive education and training for work directly related to their duties, covering the Act on Securing Quality, Efficacy and Safety of Products Including Pharmaceuticals and Medical Devices (the Pharmaceuticals and Medical Devices Act).
In fiscal 2024, we began providing education and training to ensure we meet all legal requirements as laws and regulations become increasingly diverse and complex.
Main Compliance Education and Training Implemented in Fiscal 2023
| Main Items Implemented | |
|---|---|
| Companywide training |
• Reporting results of the Fiscal 2023 Compliance Status Questionnaire • Month-long training dedicated to understanding the Code of Practice (held annually) • Distribution of materials aimed at raising awareness of the Pharmaceutical and Medical Devices Act (Fifth Edition) |
| Rank-based training |
• Training regarding the Guidelines for Sales Information Provision Activities for Ethical Drugs • Training regarding proper promotion of labor management • Training regarding a psychologically safe workplace • Promoting new compliance that is stronger and more controlled • Promoting management in accordance with the law in a new era • Countermeasures against power harassment (an abuse of power by superiors) to improve psychological safety in the workplace • New employee education (Management Philosophy, corporate code of conduct, etc.) |
| Departmental training | Conducted compliance-related training/education a total of 1,225 times in 152 departments, including training/education related to the Compliance Program Manual, promotional and drug information provision activities, Fair Competition Code case studies, etc. |
Compliance Status Survey
Since fiscal year 2005, we have been conducting an annual Compliance Status Questionnaire targeting all employees and workplaces. The purpose of this questionnaire is to assess the degree of awareness and the actual implementation of compliance in the workplace and among employees themselves.
The questionnaire results are compiled and analyzed, then fed back to each division and department. This information is utilized by compliance promotion officers, managers, and supervisors to educate general employees, and serves as a reference for the Legal Department's educational and awareness activities.
Moving forward, we will continue to effectively utilize the survey results to improve the workplace environment and further enhance compliance awareness.
Transparency Initiatives
Kissei Pharmaceutical established “Guidelines for Transparency in the Relationship between Corporate Activities and Medical Institutions” and “Guidelines for Transparency in the Relationship between Corporate Activities and Patient Groups” and discloses information on funding provided to medical institutions, patient groups, and other organizations.